Mass spectrometry is an analytical method of identifying the type and the number of chemical elements in a sample by measuring the mass-to-charge ratio of ions. Each spectrum represents a measure of the mass-to-charge ratio, so it can be used to plot a function of the ion signal. By knowing the spectra and the molecular mass of the ion, it is possible to determine the isotopic signature of a material - namely its elemental composition. This information can lead to finding out the chemical structure of molecules. The method of mass spectrometry consists of ionizing chemical compounds with the purpose of measuring their mass-to-charge ratios. To accurately read levels and analyze goods, vacuum pumps must be used to remove background interference and make readings more precise.
How can mass spectrometry achieve this?
Here are the main components of a mass spectrometer:
- Ionization Source
- Mass Analysis System
- Ion Detection System
The Ionization Source
In order to be manipulated by external magnetic and electric fields, molecules need to be converted to gas-phase ions. This can be achieved through a technique known as nanoelectrospray ionization. This procedure is very similar to industrial can painting. Depending on the specific requirements of each project, we can use this technique to create either positively or negatively charged ions. The technique can connect the outlet of a small-scale chromatography column straight to the mass spectrometer inlet. We use a special needle to direct the flow from the column through the system.
The Mass Analysis System
After ionization, ions are separated in accordance with their mass-to-charge ratios. There are several models of mass analyzers available today, each of them with its own advantages and drawbacks. Such trade-offs include various operational requirements, the speed of operation, and the resolution of separation. We take a close look at all these different options in the next chapter. The choice of the mass analyzer is usually directly linked to the ion detection system.
The Ion Detection System
After their separation, ions are measured. The system stores the m/z data together with the relative abundance of these ions. A mass spectrum is nothing else but the m/z ratios of the ions in a sample in relationship with their intensities. Each peak of a mass spectrum represents a unique chemical composition. The heights of these peaks are a measure of the abundance of these components in the given sample.
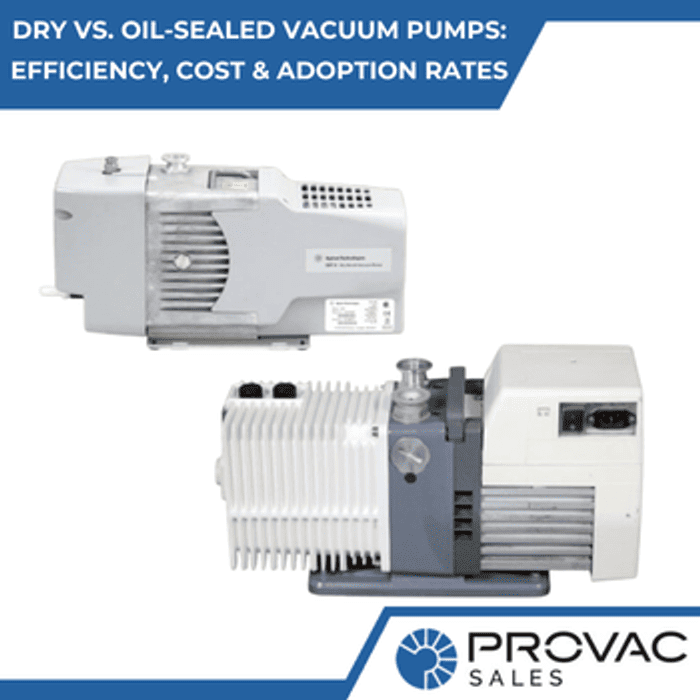
We offer a variety of pump options to be used for mass spectrometry and gas analysis. Oil-sealed vane pumps, dry scroll pumps, or dry diaphragm roughing pumps are all effective options. Additionally, high vacuum pumps help with this process as well.